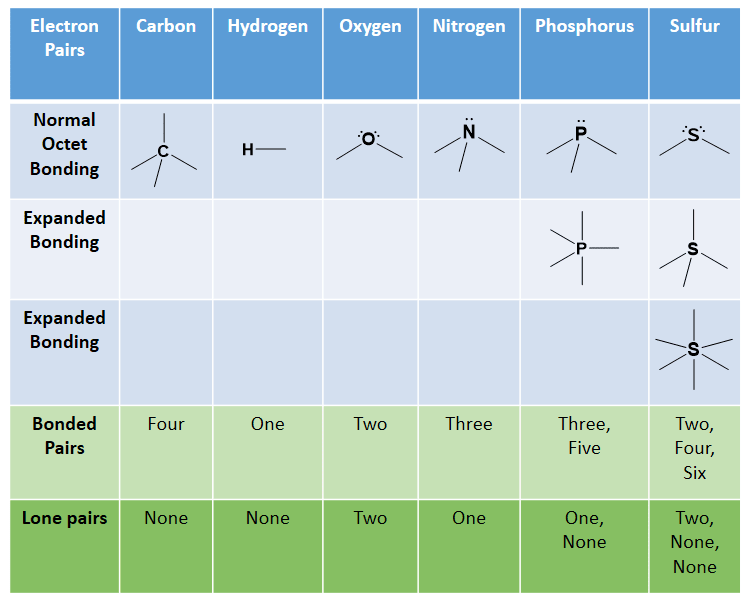
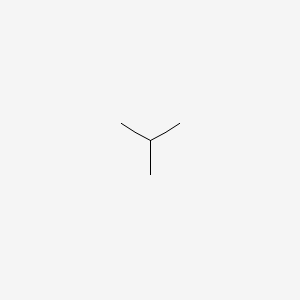Smallest Alkanes At Room Temperature

Spell out the full name of the compound.
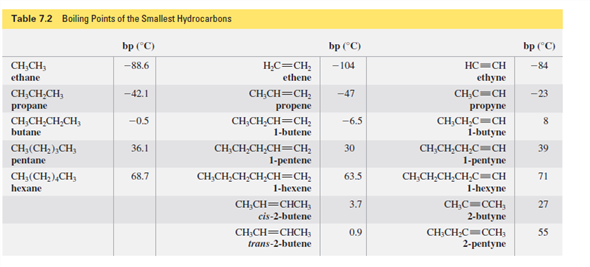
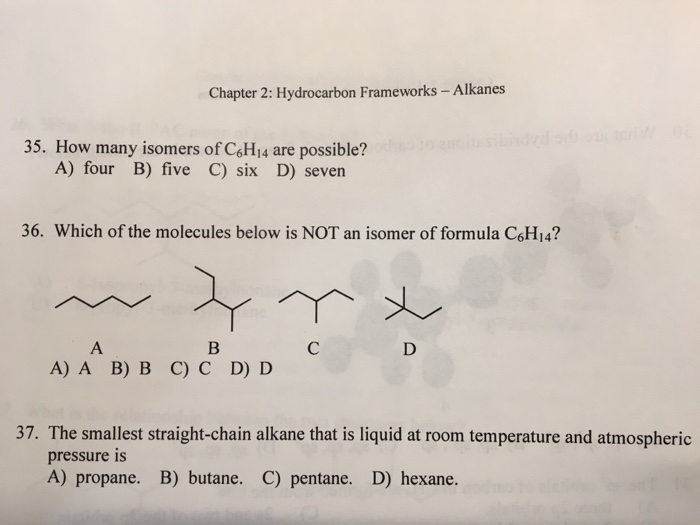
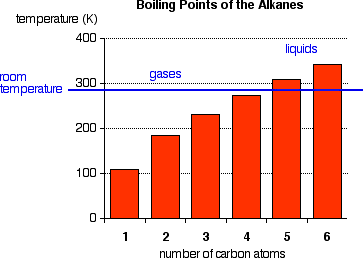
Smallest alkanes at room temperature. What is the smallest straight chain alkane that is a liquid at room temperature which is about 25 c. They have the weakest bonds holding them together. How many double covalent bonds are in an alkane. Part a what is the smallest alkane that is a liquid at room temperature which is about 25 c.
Alkanes with more than 12 carbon atoms have such strong bonds that they are solid at room temperature. The alkanes can exist as gases liquids or solids at room temperature. Check out a. Want to see the full answer.
Gas is the physical state of the smallest alkanes at room temperature. The longest continuous carbon chain of a branched chain hydrocarbon is called. Is nonane gas at room temperature. And pressure starting.
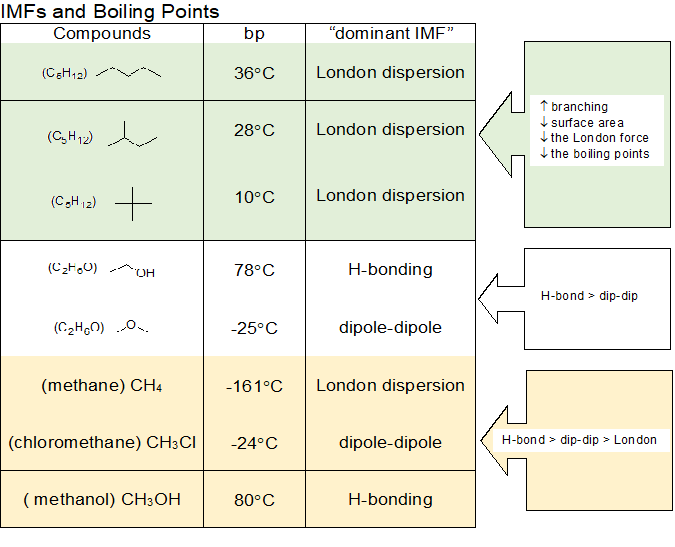
The more carbon atoms exist in an alkane the stronger its molecular bonds are. What is the smallest straight chain alkane that is a liquid at room temperature which is about 25 c. Alkanes are hydrocarbons that contain. Asked jun 27 2020.
This is why the alkanes with the smallest number of carbon atoms have the lowest vaporization points. Pentane through hexadecane are liquids. The alkanes are liquids at room temp.